Abstract
Introduction: Bruton's Tyrosine Kinase (BTK) plays a critical role in B-cell receptor (BCR) signaling, which mediates B-cell proliferation, migration, and adhesion (Rickert, 2013; Aalipour, 2013; Choe, 2016). The BCR pathway is an established therapeutic target in chronic lymphocytic leukemia (CLL), and pathway members are frequently mutated in follicular lymphoma (FL) (Byrd, 2016; Krysiak, 2017). Ibrutinib, the first-generation BTK inhibitor, has activity in FL and CLL in combination with rituximab (Fowler, 2016; Burger, 2014). BGB-3111 is a potent and specific BTK inhibitor, designed to minimize off-target inhibition of TEC and EGFR family kinases. BGB-3111 has minimal inhibitory effects against ITK and does not inhibit ITK-mediated rituximab-induced antibody-dependent cell-mediated cytotoxicity. Here we present updated interim results of an ongoing study of BGB-3111 in combination with obinutuzumab in patients with CLL/small lymphocytic lymphoma (SLL) and FL.
Methods: This is an ongoing, open-label, multicenter, phase 1b study of the combination of BGB-3111 and obinutuzumab in patients with B-cell malignancies with indication-specific expansion cohorts. Eligible patients had Eastern Cooperative Oncology Group performance status of 0-2, absolute neutrophil count >1000/μL, platelets >40,000/μL, adequate renal and hepatic function, and no significant cardiovascular disease. Growth factors and transfusions were allowed. Concomitant anticoagulation was also permitted. The primary endpoint of the expansion cohorts was response rate and duration of response using standard International Working Group (IWG) criteria for each disease. A key secondary endpoint was to evaluate the safety of the combination.
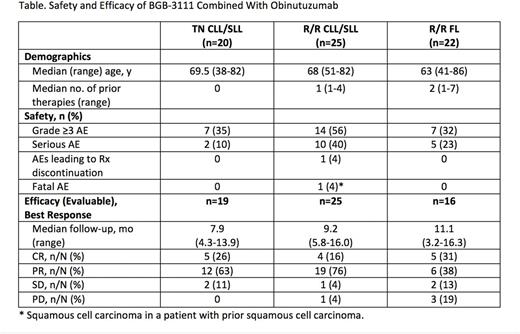
Results: As of Jun 1, 2017, 45 patients with CLL/SLL (20 pts with treatment-naïve [TN]; 25 patients with relapsed/refractory [R/R]), and 22 patients with R/R FL were enrolled. Demographic and disease characteristics are shown in the Table. Median follow-up time was 8.4 months (range, 2.6-16.0) for patients with CLL/SLL and 7.7 months (range, 0.3-16.3) for patients with FL. BGB-3111 plus obinutuzumab was well tolerated. One fatal adverse event (AE) occurred (squamous cell carcinoma in a CLL patient with prior squamous cell carcinoma). Serious AEs (SAEs) were reported in 12 (27%) CLL/SLL pts and 5 (23%) FL pts; there was only 1 SAE related to treatment (infusion-related reaction) and 1 SAE related to BGB-3111 (pneumonia). The most common (>20%) any-grade AEs for CLL patients were neutropenia (40%), contusion (22%), and thrombocytopenia; for FL patients, they were thrombocytopenia (27%), contusion (23%), fatigue (23%), and upper respiratory tract infection (23%). Grade 3-4 neutropenia was the only AE that occurred in >2 patients (22% for CLL; 14% for FL). There were no AEs of atrial fibrillation. Patients were evaluable for response if they had completed baseline and ≥1 on-treatment response assessments. Objective response rates (complete response [CR] + partial response) were 89%, 92%, and 69% in TN CLL/SLL, R/R CLL/SLL, and R/R FL, respectively, with 5 CRs in TN CLL/SLL, 4 CRs in R/R CLL/SLL, and 5 CRs in FL. Four patients (1 R/R CLL;3 FL) experienced disease progression; no instances of disease transformation occurred.
Conclusions: BGB-3111 plus obinutuzumab is well tolerated and highly active in CLL/SLL and FL. Few patients have discontinued, and responses remain durable within the larger patient sample with continued follow-up.
Quach: Amgen: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria; Takeda: Consultancy, Honoraria. Badoux: Roche: Consultancy. Prince: Millennium Pharmaceuticals, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees; Eisai: Membership on an entity's Board of Directors or advisory committees; Celgene Corporation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Research Funding. Eek: Border Medical Oncology: Employment; Ramsey Private Hospital Albury Wodonga: Membership on an entity's Board of Directors or advisory committees. Huang: BeiGene: Employment. Zhang: BeiGene: Employment. Wang: BeiGene: Employment. Hedrick: BeiGene: Employment. Novotny: BeiGene: Employment. Flinn: Constellation: Research Funding; Curis: Research Funding; Gilead: Research Funding; Portola: Research Funding; Pharmacyclics: Research Funding; Trillium: Research Funding; KITE: Research Funding; Janssen: Research Funding; Calithera: Research Funding; Incyte: Research Funding; Agios: Research Funding; Infinity: Research Funding; Acerta: Research Funding; Celgene: Research Funding; Genentech: Research Funding; Janssen: Research Funding; Pharmacyclics LLC: Research Funding; Verastem: Research Funding; AbbVie Company: Research Funding; TG Therapeutics: Research Funding; Takeda: Research Funding; Seattle Genetics: Research Funding; Pfizer: Research Funding; Merck: Research Funding; Novartis: Research Funding; Beigene: Research Funding; Forty Seven: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal